Contributors: Vengatesa Prasanna, Associate Director – Projects & Rajalakshmi Venugopal, Associate Director – IP [Oct 2025]
Explore how AI, biotech R&D, and data-driven innovation are transforming global stroke care and accelerating life sciences breakthroughs.
A Data-Driven Wake-Up Call: Stroke Research Enters a New Era
In early 2025, the conversation around stroke care reached a critical inflection point. New analyses reminded the world that each minute of untreated large-vessel ischemic stroke destroys roughly 1.9 million neurons—an irreversible neurological loss that redefines the urgency of intervention [1].
The economic impact is equally devastating. According to the U.S. Centers for Disease Control and Prevention (CDC), the national cost of stroke reached approximately $56.2 billion for 2019–2020, including healthcare expenses, medications, and lost productivity [2].
Despite advances in stroke awareness, delays remain severe. A multicenter review found median door-in–door-out (DIDO) times exceeding 170 minutes, well above the ≤120-minute target recommended for interhospital transfer [3]. Each minute saved is a chance to protect cognition, independence, and life itself.
As Mikael Dolsten, Chief Scientific Officer at Pfizer, emphasized in 2025, “Putting human and artificial intelligence next to one another can play such a tremendous role” in accelerating discovery and care integration [4].
AI in Drug Discovery: Accelerating the Race Against Time
Between 2023 and 2025, artificial intelligence evolved from promise to practice in neurological drug discovery. Machine learning models now predict molecular efficacy, blood-brain-barrier permeability, and ischemic outcomes with measurable precision.
Key Trends Driving Stroke Therapeutics Innovation
| Focus Area | 2023–2025 Developments | Example Companies |
| AI-driven target identification | Deep-learning applied to cerebrovascular datasets identifying new neuroprotective pathways | Insilico Medicine, BenevolentAI |
| In-silico clinical simulation | Virtual patient cohorts model clot resolution and reperfusion timelines | Novartis, Roche |
| Precision medicine | Integration of proteomic and genomic markers for personalized antithrombotic therapies | Sanofi, Johnson & Johnson |
| Regenerative neurobiology | Computational models accelerate stem-cell and neurorepair research | AbbVie, AstraZeneca |
Fiona Marshall, President of Biomedical Research at Novartis, noted in 2025 that the company is “using AI end-to-end across all the things that we’re doing,” underscoring how digital intelligence now underpins every stage of discovery [5].
Preclinical Development and the Rise of Predictive Analytics
Translating efficacy from animal models to human outcomes remains a notorious challenge in stroke therapeutics. Historically, more than 80% of neuroprotective agents have failed in late-stage trials, largely due to species differences and trial design variability.
Modern AI-enabled preclinical analytics mitigate this attrition by integrating imaging biomarkers, transcriptomics, and cellular response data. Predictive platforms can now flag compounds most likely to translate effectively before costly clinical investment.
The Biomedical Advanced Research and Development Authority (BARDA) has advanced this agenda through its DRIVe Accelerator and innovation programs, which fund early-stage health-security technologies and predictive modeling tools for neurological emergencies [6]. These collaborative models demonstrate how data-sharing and simulation shorten preclinical-to-clinical translation cycles.
Regulatory Strategy: The AI-Ready Review Process
Regulators have moved rapidly to match scientific innovation. On January 6, 2025, the U.S. FDA released a risk-based framework guiding how sponsors can evaluate and demonstrate the credibility of AI models used in regulatory submissions [7]. The framework lays out principles for transparency, traceability, and model validation.
In parallel, the agency announced internal AI pilots to assist reviewers in managing complex data streams from adaptive clinical trials [8]. These actions mark a turning point: AI is no longer an experimental supplement to regulatory science—it is a recognized evidentiary tool.
Patrizia Cavazzoni, Director of the FDA’s Center for Drug Evaluation and Research (CDER), summarized the balance succinctly at a 2025 stakeholder meeting: speed and safety must evolve together through trustworthy digital evidence.
Manufacturing and Technology Transfer: Precision at Scale
Once a stroke therapy shows efficacy, manufacturing agility and knowledge transfer become the next bottlenecks. Traditional processes relied heavily on static documentation and manual oversight. Today, digital twins, advanced analytics, and blockchain-secured traceability are reshaping how scale-up occurs across facilities.
Large pharmaceutical organizations—including Roche, Sanofi, and Dr. Reddy’s Laboratories—have publicly discussed digitalization of manufacturing and supply-chain processes. Roche, for example, has deployed digital-twin initiatives within diagnostics and bioprocessing to enhance predictability and reduce deviation rates [9]. Parallel research collaborations have explored blockchain frameworks for pharmaceutical supply-chain integrity, some involving Indian manufacturers such as Dr. Reddy’s Laboratories [10].
These examples illustrate a broader trend rather than isolated pilots: connected manufacturing ecosystems enable reproducibility, compliance, and scalability without compromising patient safety.
Biotech R&D and Life Sciences Data Services: The Convergence Point
By 2025, data unification has become the cornerstone of successful life-sciences innovation. Organizations are consolidating experimental, clinical, and literature data into harmonized ontologies that allow rapid querying and cross-domain analytics.
Strategic Advantages of Integrated Data Infrastructure
- Accelerated hypothesis generation through multimodal data correlation
- Cross-trial endpoint harmonization improving statistical power
- Comprehensive IP landscaping for crowded neurological-therapeutic spaces
In 2025 major organisational research roadmaps highlighted data harmonization as “the single most impactful differentiator” for accelerating neuroscience R&D. This echoes industry consensus that data curation, indexing, and abstracting are now strategic assets, not administrative chores.
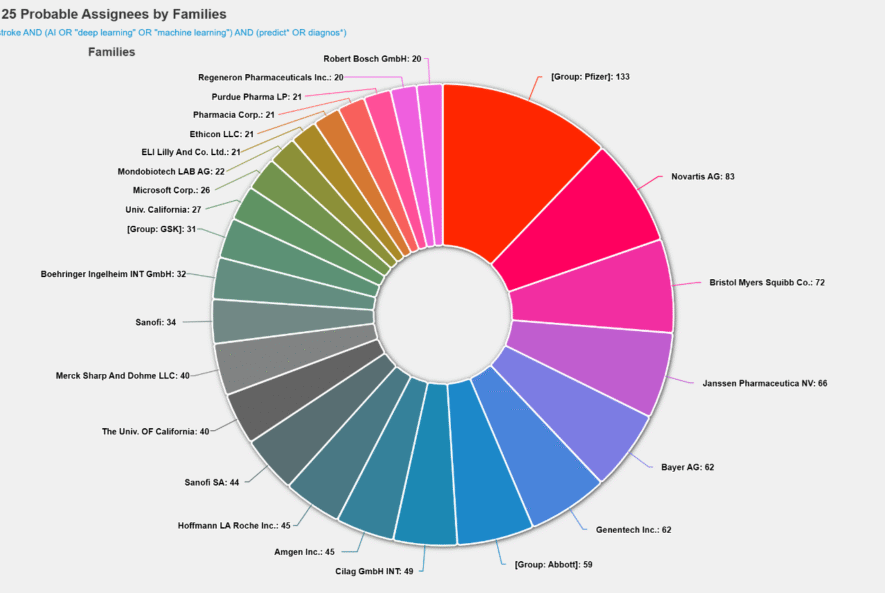
Top Assignees in AI-Related Patents for Stroke Prediction
The chart illustrates the top 25 patent assignees actively innovating in the field of AI-driven stroke prediction and diagnosis. The search strategy combined keywords related to stroke, artificial intelligence, machine learning, and prediction/diagnosis, capturing a broad spectrum of inventions from imaging algorithms to predictive analytics and wearable monitoring systems.
From the analysis, Pfizer leads significantly with 133 patent families, followed by Novartis (83) and Bristol Myers Squibb (72), highlighting strong pharmaceutical engagement in AI-based stroke research and outcome modeling. Janssen Pharmaceuticals (66), Bayer (62), Genentech (62), and the Abbott Group (59) also appear as key contributors, indicating a trend toward integrating AI into drug discovery, stroke risk stratification, and patient management systems.
This growing patent activity reflects the convergence of pharmaceutical R&D, medical imaging, and artificial intelligence collectively driving innovation toward earlier detection, better prediction models, and improved stroke outcomes.
Top Jurisdictions for AI-Related Patents in Stroke Prediction
It is equally important to highlight the top 10 jurisdictions leading in patent filings related to AI and machine learning applications for stroke prediction and diagnosis. The analysis reveals that the United States dominates the landscape with over 16,000 patent families, underscoring its strong focus on medical AI and digital health innovation.
In Asia, Japan (8,287) and China (6,485) emerge as strong contributors, focusing on AI-assisted imaging, wearable sensors, and predictive analytics for stroke risk assessment. India (3,580) also shows a promising rise in filings, indicating an expanding ecosystem of AI-based healthcare startups and research collaborations addressing stroke prediction and preventive care.
Other active regions include Australia (7,308), Canada (5,884), Germany (3,935), Israel (3,499), and Mexico (3,787) demonstrating how innovation in stroke-related AI is spread across continents.
Overall, this distribution highlights a global momentum in integrating AI with neurology and diagnostics, with India and other emerging economies becoming vital players in advancing accessible, technology-driven stroke care.
Saturo Global: The Data Backbone Behind Stroke Innovation
Behind every AI-powered breakthrough lies structured, interoperable data. Saturo Global partners with biotech and pharma organizations to transform raw scientific information into actionable intelligence.
Core Offerings
- Data Curation: Normalizes heterogeneous experimental and clinical datasets for AI and statistical modeling.
- Indexing & Abstracting: Links stroke-specific literature and trial results across public and proprietary repositories.
- Strategic Patent Support: Maps intellectual-property landscapes to inform investment in next-generation neuroprotective and thrombolytic compounds.
Clients adopting Saturo Global’s data-governance solutions have reported measurable R&D efficiencies, including reduced duplication of literature screening and faster regulatory-dossier compilation. These operational gains echo the Roche data-science insight from 2025: “Our AI models are only as good as the data that feeds them”—a principle that defines competitive advantage in modern R&D.
For any service related to your data-driven processes, please do not hesitate to contact us and do connect on LinkedIn.
Looking Ahead: Partnerships That Redefine the Clock
Stroke innovation demonstrates an unambiguous truth: time and data are now inseparable variables in saving lives. The convergence of AI analytics, real-world data integration, and adaptive manufacturing will continue to compress discovery-to-therapy timelines.
The next phase of biotech and pharma collaboration will favour ecosystem partnerships over proprietary silos. Shared data standards, interoperable platforms, and transparent regulatory engagement will underpin the next generation of drug-development alliances.
As World Stroke Day 2025 reminds us, every minute counts. The companies that act with speed, clarity, and collaboration will lead the future of brain-health innovation—and redefine what “fast” means in science.
References
- Saver JL. Time is Brain—Quantified. Stroke. 2006;37(1):263-266.
- Centers for Disease Control and Prevention. Stroke Facts. Updated 2024.
- Mowla A et al. Door-In Door-Out Times for Acute Ischemic Stroke Transfers. Stroke. 2022;53(11):3441-3448.
- Dolsten M. Quoted in PharmaVoice, April 2025 interview, “AI and the Next Frontier of Biopharma.”
- Marshall F. Interview, PharmaVoice, March 2025, “Novartis Uses AI End-to-End Across R&D.”
- Biomedical Advanced Research and Development Authority (BARDA). DRIVe Accelerator Overview. HHS.gov, 2024.
- U.S. Food and Drug Administration. Proposed Framework for the Credibility of AI Models for Regulatory Decision-Making. Jan 6 2025.
- U.S. FDA Newsroom. FDA Pilots AI Tools to Enhance Reviewer Efficiency. Feb 2025.
- Roche Group. Digital Twin Applications in Biomanufacturing and Diagnostics. Roche.com, 2024.
- World Economic Forum. Blockchain Solutions for Pharmaceutical Supply Chains. 2024 Report.
- PatBase- Global Patent database



