The next frontier in Keratoconus R&D
Contributed by: Rajalakshmi Venugopal
Keratoconus is shifting from “watchful waiting” to “precision stabilization.” The anchor is epithelium-off corneal cross-linking (CXL), which shows long-term efficacy in decreasing disease progression, while genetics and biomechanics are opening doors to earlier risk detection and smarter trial design.
A human moment
In 2016, the first FDA approval of CXL gave progressive keratoconus patients a path to halt worsening vision; by 2024, multiple studies affirmed durable stabilization and renewed work capacity for many patients who otherwise might have trended toward corneal transplantation. That evidence base has reframed care pathways and created a platform for innovation.
Why keratoconus merits R&D investment now
- Treatments and breakthroughs: Epi-off CXL remains the validated baseline with long-term efficacy; evolving protocols for thin corneas and investigational epi-on approaches aim to broaden eligibility and reduce morbidity.
- Data Analytics and Artificial Intelligence: Polygenic risk models and multitrait PRS can add predictive power beyond imaging alone, supporting earlier intervention and trial enrichment.
- Technology Transfer: Clinically relevant biomechanics, tomography, and standardized CXL metrics enable translational models and reproducible endpoints for Biotech R&D and Pharma Innovation.
A simple analogy
The cornea functions like a geodesic dome. In keratoconus, weakened “struts” cause buckling. CXL reinforces the lattice to halt collapse; genetics, imaging, and biomechanics help identify which struts are failing earliest, while surgical bridges buy time in advanced phases.
Biotech R&D across the value chain
Drug discovery: biological targets and hypotheses
- Matrix and oxidative stress biology: CXL’s mechanism highlights stromal collagen and reactive oxygen species biology; related stromal pathways remain relevant for pharmacologic strategies aimed at remodeling or stiffening.
- Genetics to targets: Recent comprehensive reviews and GWAS-driven work consolidate risk loci and enable polygenic scoring, pointing to extracellular matrix and signaling pathways as investigational targets.
- Actionable takeaway: Prioritize target validation in stromal keratocytes and extracellular matrix pathways, using endpoints that map to corneal shape and stiffness readouts used clinically.
Preclinical development: models and metrics
- Thin-cornea protocols: Systematic evidence supports modified CXL approaches for corneas less than 400 μm (e.g., contact-lens–assisted, hyperosmolar riboflavin, adjusted fluence), expanding the evaluable population for translational research.
- Biomechanical endpoints: Cross-linking increases stromal stiffness via ROS-mediated cross-links; emerging device-based biomechanics and tomography-derived surrogates can serve as preclinical-to-clinical bridges.
- Combination paradigms: Intrastromal corneal ring segments (ICRS) combined with CXL improve corneal shape and vision in selected phenotypes, offering comparator or combination frameworks for future therapies.
- Actionable takeaway: Build preclinical packages around reproducible stromal models and biomechanical function assays aligned to clinical tomography/topography endpoints.
Regulatory strategy: device versus disease-modifying therapy
- Current baseline: Epi-off riboflavin/UVA CXL is established and FDA-cleared in the United States; transepithelial (epi-on) approaches remain under investigation and require longer-term data for regulatory decisions.
- Drug or biologic pathways: Disease-modifying pharmacology aimed at stromal remodeling will need durable changes in clinically meaningful endpoints such as Kmax and vision, supported by validated biomechanical measures.
- Companion tools: Polygenic risk scores can enhance cohort selection and progression prediction, strengthen enrichment strategies and potentially support companion diagnostic development.
- Actionable takeaway: For device–drug combinations, predefine contribution-of-components and align structural, functional, and biomechanical endpoints early to reduce regulatory risk.
Manufacturing and scale-up: specialized modalities
- Quality by design: Photochemical performance of riboflavin/UVA systems, oxygen availability, and dose delivery must be tightly controlled and verified batch-to-batch.
- Future biologics and cell therapies: If stromal-targeting agents or cell-based strategies advance, chemistry, manufacturing, and controls should centre on stromal integration and biomechanical function assays that correlate with clinical endpoints.
- Actionable takeaway: Standardize biomechanical functional testing and tomography-mapped metrics as release criteria to streamline Technology Transfer.
What leading teams are doing already
- Anchor on validated care: Programs position epi-off CXL as the backbone to halt progression and reduce keratoplasty trajectories.
- Extend eligibility responsibly: Thin-cornea adaptations increase the treatable population while informing protocol optimization and patient segmentation.
- Bring genetics into analytics: Multitrait PRS augments prediction beyond imaging, supporting risk-aligned screening and trial design.
- Use surgical bridges strategically: Bowman layer transplantation offers multi-year stabilization in advanced disease, preserving function and delaying grafts where appropriate.
Treatments and breakthroughs: status by 2025
- Epithelium-off CXL: Long-term efficacy and safety across multiple studies; remains the standard stabilization strategy.
- Thin-cornea CXL: Systematic review supports modified protocols for corneas below 400 μm, though guidance and harmonization are evolving.
- Transepithelial (epi-on) CXL: Early results and expert commentary suggest potential, but more long-term data are needed for regulatory-grade conclusions.
- Surgical bridges: Bowman layer transplantation shows significant keratometry reductions and favorable multi-year success in advanced keratoconus in a systematic review.
Rapid reference table: interventional options
| Modality | Core aim | Key evidence to date | Where it fits |
| Epithelium-off CXL | Halt progression | Long-term efficacy and safety; FDA-cleared protocol baseline | First-line stabilization in progressive cases |
| Thin-cornea CXL variants | Expand eligibility | Systematic review supports modified protocols; harmonization ongoing | Progressive disease with corneas <400 μm |
| ICRS ± CXL | Improve corneal shape and vision | Clinical experience and reports show gains in selected phenotypes; often combined with CXL | Bridge therapy in phenotype-guided cases |
| Bowman layer transplantation | Stabilize advanced ectasia | Systematic review shows significant keratometry reductions and multi-year success rates | Advanced disease to defer graft |
Data Management, Data Analytics, and AI opportunities
- Life Sciences Data Services: Harmonize tomography, topography, endothelial counts, and demographic risk with genomic features to build progression risk models and responder profiles.
- Data Analytics and Artificial Intelligence: Train predictive models that combine PRS with imaging-derived metrics to trigger earlier, personalized intervention and power-efficient trials.
- Biotech R&D: Use clinically aligned biomechanical and topographic readouts as translatable endpoints in preclinical screening.
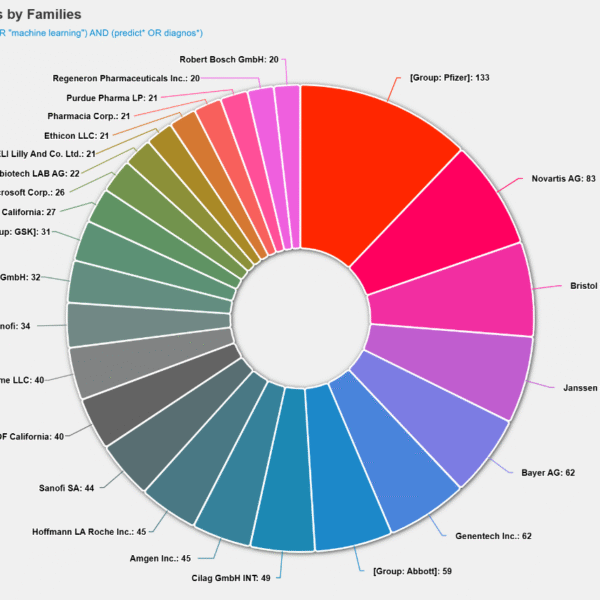
Recent patent innovations focused on keratoconus
Keratoconus is a progressive eye disorder that causes the cornea to thin and bulge into a cone-like shape, resulting in distorted vision and, in severe cases, blindness. Recent advancements in research and patent innovations have focused on improving diagnosis, treatment, and modeling of this condition. In 2025, several significant patent publications have emerged in this field, for instance, RU2846471 C1 introduces a breakthrough in biotechnology by developing a three-dimensional keratoconus model using living cells and trace elements like zinc, copper, and iron to simulate disease conditions for better therapeutic testing. Treatment innovations such as WO2025181000 A1 propose enhanced corneal cross-linking methods using benzalkonium chloride and EDTA to increase safety and comfort during procedures. Natural compound–based therapies are also emerging; CN118845755 A utilizes soy isoflavone for corneal repair with minimal toxicity, offering a patient-friendly eye drop formulation. On the diagnostic front, CN117417997 A identifies specific genetic markers linked to keratoconus, enabling earlier and more precise detection. Novel medical devices like IN202521059476 A describe an innovative therapeutic contact lens incorporating a gel to reshape the cornea and promote healing non-surgically. In the area of disease assessment, RU2024100421 A introduces a biomechanical formula for evaluating corneal rigidity and classifying disease stages with improved accuracy. Finally, surgical innovations such as RU2841923 C1 present laser-assisted insertion of donor corneal segments, enhancing visual outcomes and structural stability. Collectively, these patents signify a comprehensive shift toward personalized, less invasive, and biologically informed management of keratoconus.
How Saturo Global empowers R&D teams
- Data Curation & Management: Integrate and standardize imaging, biomechanics, and genomic datasets into analysis-ready layers; normalize Kmax, thinnest pachymetry, demarcation line depth, endothelial metrics, and PRS variables for multi-site studies.
- Indexing & Abstracting: Continuously index global peer‑reviewed studies, clinical protocols, and device–procedure updates in keratoconus to accelerate discovery and Technology Transfer.
- Strategic Patent Support: Map the competitive landscape around cross-linking protocols, adjuncts, and surgical bridges; identify whitespace and monitor overlapping claims across jurisdictions to inform portfolio strategy.
- Data Visualization: Deliver executive-ready dashboards that translate complex keratoconus datasets into clear insights for Treatments and breakthroughs, trial readiness, and pipeline fit across Biotech R&D and Pharma Innovation.
Actionable takeaways for 2025
- Build a precision front door: Deploy PRS- and imaging-driven risk scoring to triage earlier intervention and enrich trials.
- Treat stabilization as the benchmark: Use epi-off CXL outcomes as the reference when evaluating adjuncts and next-gen modalities.
- Standardize translational endpoints: Anchor preclinical go/no-go decisions to biomechanical and tomographic metrics that map to clinical endpoints.
- Plan for combination pathways: Define contribution-of-components for device–drug strategies and align endpoints early for regulatory efficiency.
Conclusion
The next era of keratoconus care is precision-first and partnership-driven. Organizations that integrate rigorous Data Management, translational biomechanics, and AI-enabled risk stratification will move faster from insight to indication, shaping disease-modifying strategies that preserve vision and productivity. Strategic, data-driven collaborations will define leadership in 2026 and beyond.
Meta description: Keratoconus is the next frontier in biotech R&D: validated cross-linking, genetics-driven risk, and biomechanics powering precision therapy.
References
- Greenstein SA, Hersh PS. 2024. Update on corneal crosslinking for keratoconus and corneal ectasia. Current Opinion in Ophthalmology. doi:10.1097/ICU.0000000000001056
- Hafezi F, et al. 2024. Corneal cross-linking. Current Opinion in Ophthalmology/Review article overview on photochemical mechanism and stiffness effects. Accessed via ScienceDirect listing.
- Systematic review and meta-analysis on CXL in corneas <400 μm. 2023–2024. Journal page on ScienceDirect; modified protocols and safety considerations summarized on article page.
- Review of Ophthalmology. 2023. What’s Next for Corneal Cross-Linking. Expert commentary on epi-on approaches and combination strategies; emphasizes need for long-term data.
- Cerván‑Martín M, et al. 2024. Comprehensive Evaluation of the Genetic Basis of Keratoconus. International Journal of Molecular Sciences. doi available on article page (PMCID provided).
- Lee SSZ, et al. 2024. Polygenic Prediction of Keratoconus and its Measures: Multitrait PRS performance. ScienceDirect abstract page; Ophthalmology.
- PubMed entry for PRS validation study. 2024 online/2025 issue. Human Molecular Genetics. Model improved AUC when added to corneal measures.
- De Clerck EEB, et al. 2023. Bowman Layer Transplantation for Treating Keratoconus: A Systematic Review. Journal of Clinical Medicine. doi:10.3390/jcm12062176; PMCID available.
- Clinical context on combination strategies including ICRS and CXL; expert interviews and practice perspectives. EyeWorld. 2024–2025 coverage.
- Clinical Ophthalmology and practice reports on ICRS with CXL in selected phenotypes; overview accessed via Dovepress portal.